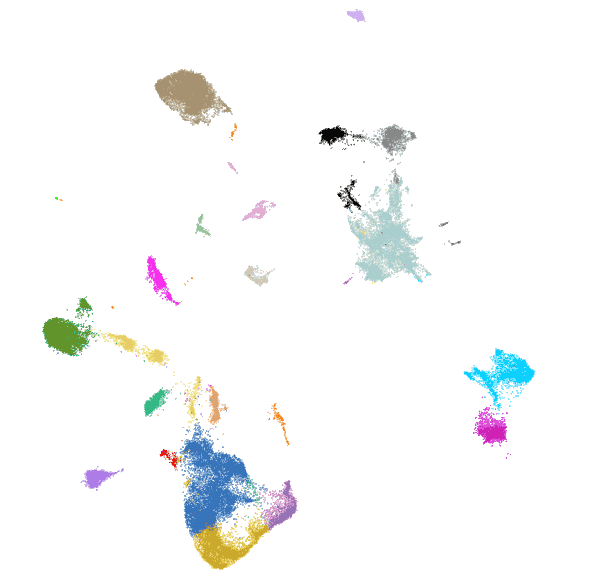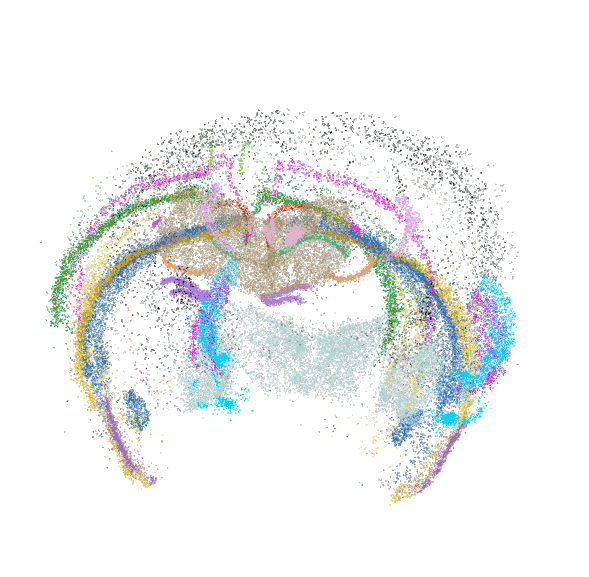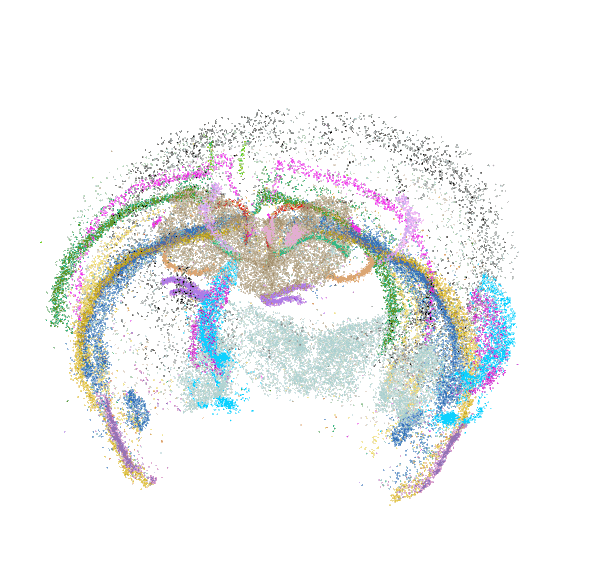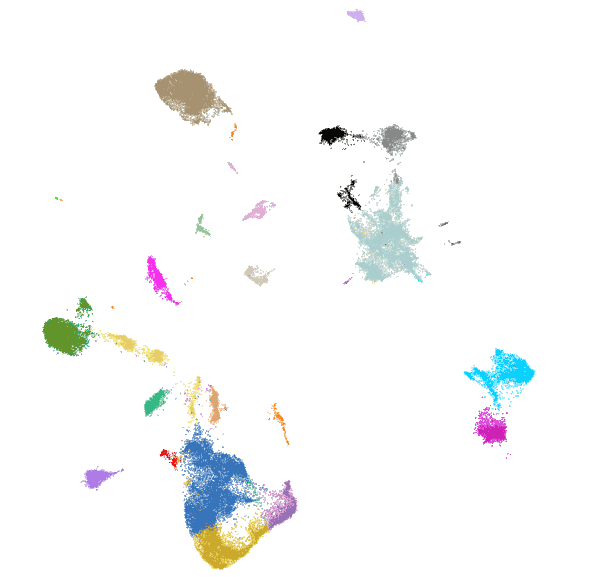Memory circuits and neuro-adipose communication
-
Memories are stored in sparsely distributed neural ensembles called “engrams”. These are ensembles of cells that fire together during a learning experience and later reactivate during recall. Each engram forms an integrated network spanning multiple brain regions (such as the hippocampus, amygdala, and cortex) and even includes non-neuronal support cells like astrocytes.
One of our fundamental questions is why certain neurons get recruited into a new memory circuit while neighboring neurons do not. Emerging evidence shows neurons with inherently higher excitability have an advantage in being selected, and indeed they undergo lasting structural and molecular changes once a memory is formed. Critically, silencing these specific “engrams” can block retrieval of the memory, whereas reactivating them later can bring it back even in the absence of the original cue.
Continuous Remodeling. Unlike a fixed snapshot, memory circuits are continuouslyremodeled by time and experience. During systems consolidation, a hippocampal trace is progressively re-expressed in distributed cortical hubs—a process still debated in detail. The memory’s representation migrates across regions, preserving content while rewiring circuitry. Moreover, each time we recall a memory or learn something new related to it, the neural ensemble can be further updated and reconfigured. In this way, memories are continuously refined (or sometimes distorted) by subsequent experiences.
To unravel these processes, we are developing high-throughput tools to map and manipulate memory circuits at single-cell resolution. We apply these techniques in models of healthy aging and in neurodegenerative disease to pinpoint how memory networks change when the brain ages or becomes ill. In disorders such as Alzheimer’s disease, for example, the reactivation of engram cells is impaired, contributing to memory loss. By understanding how engrams assemble, persist, and fail, we aim to identify new strategies to preserve memories and treat dementia.
-
Brain–Fat Signals. The nervous system regulates energy homeostasis by controlling both food intake (centrally) and the mobilization and storage of energy (peripherally). Adipose tissue—once viewed merely as an energy reservoir—is now recognized as a dynamic endocrine organ that actively participates in metabolic regulation. Through a bidirectional neuro-adipose dialogue, the brain and fat continuously exchange signals to coordinate energy balance. Sympathetic fibers innervating adipose tissue release norepinephrine, triggering lipolysis and thermogenesis, while sensory fibers embedded in fat relay information on lipid reserves, inflammation, and even mechanical stretch back to the brain. Together, this feedback loop helps maintain whole-body energy homeostasis.
Plasticity and Imbalance. The neuro-adipose circuit is remarkably adaptable. Cold exposure amplifies sympathetic activity and can induce new nerve growth within adipose tissue, enhancing its capacity for burning fuel and generating heat. In contrast, chronic overeating and inflammation impair this circuitry, leading to adipose neuropathy—a degenerative state that predisposes fat tissue to insulin resistance and metabolic dysfunction.
These dynamics raise a critical question: why do adipose nerves flourish under certain conditions yet degenerate under others? We hypothesize that distinct sub-circuits of sympathetic and sensory nerves—defined by their molecular identities and projection patterns—are differentially engaged by metabolic stimuli such as fasting, exercise, cold, or overfeeding. One pathway may drive thermogenesis during cold exposure; another may mobilize fuel during energy scarcity. Deciphering how these fibers integrate nutritional, hormonal, and immune signals will clarify how the nervous system orchestrates lipolysis, glucose uptake, and heat production.
To address these questions, we are developing an integrative toolkit that combines neural-circuit tracing with single-cell and spatial transcriptomics. Using these methods, we are constructing a comprehensive wiring-and-molecular atlas of the neuro-adipose interface. This map will reveal how connections form, remodel, and degenerate, allowing us to pinpoint molecular checkpoints that could be targeted to therapeutically rewire fat innervation. Ultimately, these insights will pave the way for new strategies to treat metabolic disorders.
Approaches
To dissect the neuronal circuits that govern memory formation and adipose-tissue metabolism, we follow a continuous workflow that begins with systematic observation, proceeds through targeted intervention, and culminates in data-driven prediction.
By integrating single-cell and spatial omics with patient-derived specimens, genetically engineered animal models, and a range of behavioral assays, we map intercellular communication—from synaptic signaling within memory circuits to neural innervation of adipose depots.
By developing autonomous agent architectures that transform these multimodal data streams into actionable predictions for precision-medicine applications. Leveraging multi-omics profiling, CRISPR-mediated perturbations, neural–organ connectomics, alongside agent-driven insights, we elucidate how disruptions in cellular networks drive cognitive decline and metabolic disease, paving the way for next-generation therapeutics in precision endocrinology.
Each dot is an individual neuron, color-coded by its molecularly defined cell type.

Spatially resolved molecular profiling of hippocampus using MERFISH

Spatially resolved molecular profiling of amygdala using MERFISH

Spatially resolved molecular profiling of prefrontal cortex using MERFISH

Brown adipose tissue shows morphological dynamics in mice live in various temperature conditions.

Aldh1a1+ adipocytes (red) and Ucp1+ adipocytes (green) in brown adipose tissue